STSM grant period 4 is now open
Osteoarthritis (OA) is the most common form of arthritis and the single most common cause of pain and physical disability in older adults.
An estimated 10% to 15% of all adults aged over 60 have some degree of OA, with prevalence being higher among women than men and likely representing underreporting which is common in many disease prevalence studies.
Despite the growing OA epidemic and major socio-economic impact, the population is facing a staggering lack of disease-modifying therapies that can bring symptomatic relief and preserve joint function by preventing cartilage and joint degeneration and thus delaying OA progression.
The research specifically aimed at OA management in Europe is scattered and not strategically coordinated, although several networks have OA partly in focus, it is a minor part of their agenda and lacks the focus and dedicated commitment to coordinate progress.
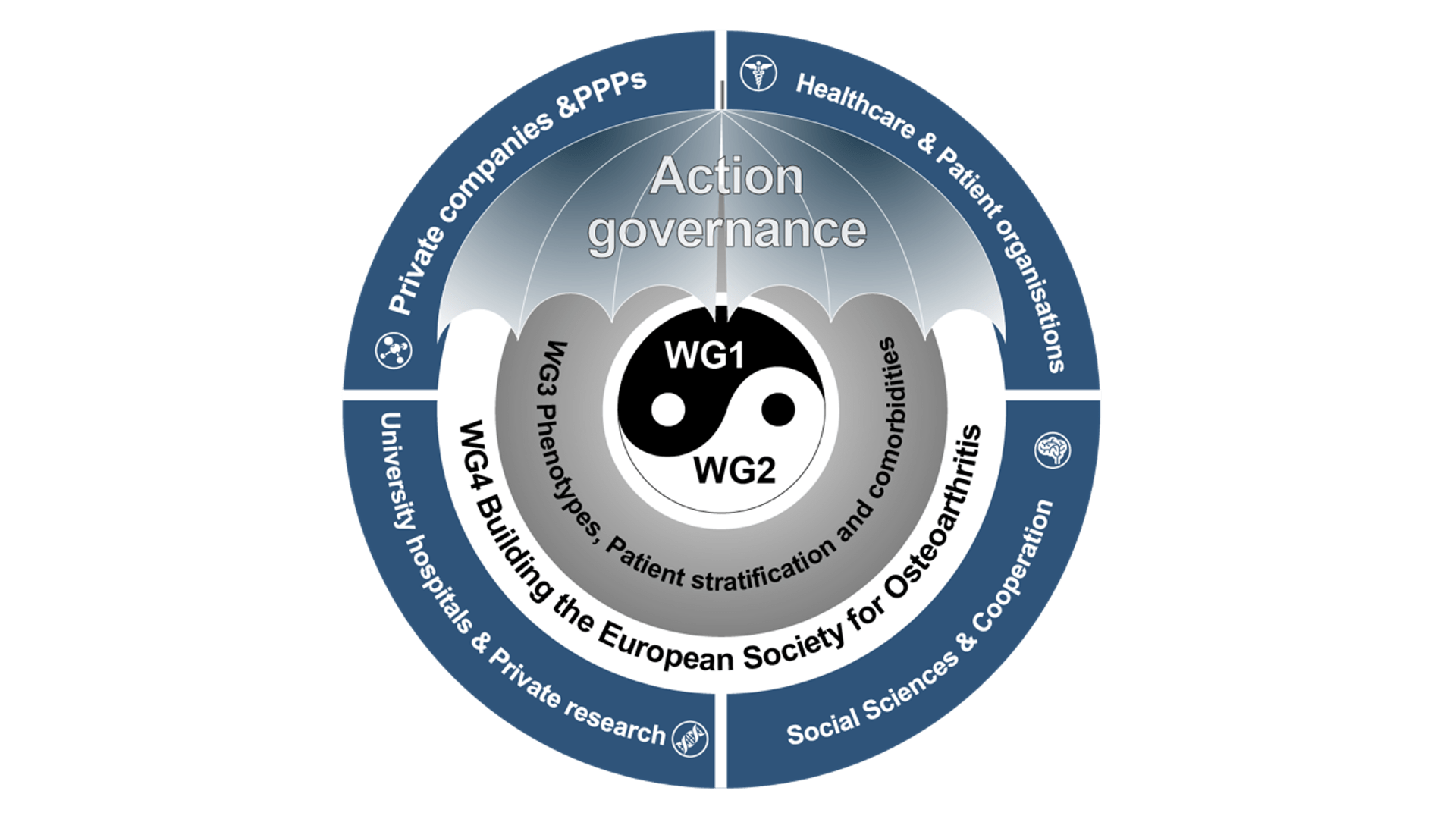
The main aim of EU-netwOArk is to set up the European Society for Osteoarthritis (EUSOA), with three major stakeholder groups:
Patients, Clinicians and Researchers (from academia and industry)


Patient organisations involved with netwOArk
Arthrose Forum Austria (Österreich) https://www.arthroseforumaustria.at/
Arthrose Kompetent Netzwerk TEPFIT e.V. (Deutschland) https://tepfit.eu/
Arthritis Ireland (Éire) https://www.arthritisireland.ie/
Norsk Revmatikerforbund (Norge) https://www.revmatiker.no/
Osteoarthritis Foundation International (Espania) https://www.oafifoundation.com/en/
Osteoartrit Association (Türkiye) website to come
RheumaCura Foundation (Schweiz) https://www.rheumacura.ch/de/
Rheumatikerförbundet (Sverige)https://reumatiker.se/
Stichting ReumaNederland (Nederland) https://reumanederland.nl/

Survey of OA patients across Europe to assess their level of understanding of their condition and determine what they know about treatment guidelines.
The aim of this study is to determine what the knowledge about osteoarthritis is among people with osteoarthritis, what they themselves can do in case of complaints of osteoarthritis and what they can expect from healthcare providers.
You can translate the survey in another language with the drop down menu in the left upper corner of the survey.
On the 5 June 2023 the institutional research review board of the Erasmus MC University Medical Center in Rotterdam, the Netherlands reviewed the protocol and the questionnaire.
The Institutional research review board Erasmus MC (hereafter the Committee) of Rotterdam, The Netherlands, has reviewed the above mentioned research proposal. As a result of this review, the Committee confirms that the rules laid down in the Medical Research Involving Human Subjects Act (also known by its Dutch abbreviation WMO), do not apply to this research proposal.”
https://erasmusmcsurvey.erasmusmc.nl/netwoark/ls/index.php/617132
FAQ Survey NetwOArk
The NetwOArk Working Groups
The COST Action (CA21110) will allow us to start the process of building such a European Society, with the aim of coordinating and stimulating more interdisciplinary and transdisciplinary research, technological development and translation of the results to the clinic aimed at improving the quality of life of those affected by OA in Europe.
The areas to be addressed in this Action are Primary prevention, Diagnostics, Treatment, Interaction (comorbidities) and Care Management. The EU-netwOArk aims to boost new scientific breakthroughs on these five main OA themes.